Enhanced permeation5
- At half the strength of other clobetasol products, Impoyz and its formulation showed lower systemic exposure compared to Temovate® (clobetasol propionate cream) Cream, 0.05%.2
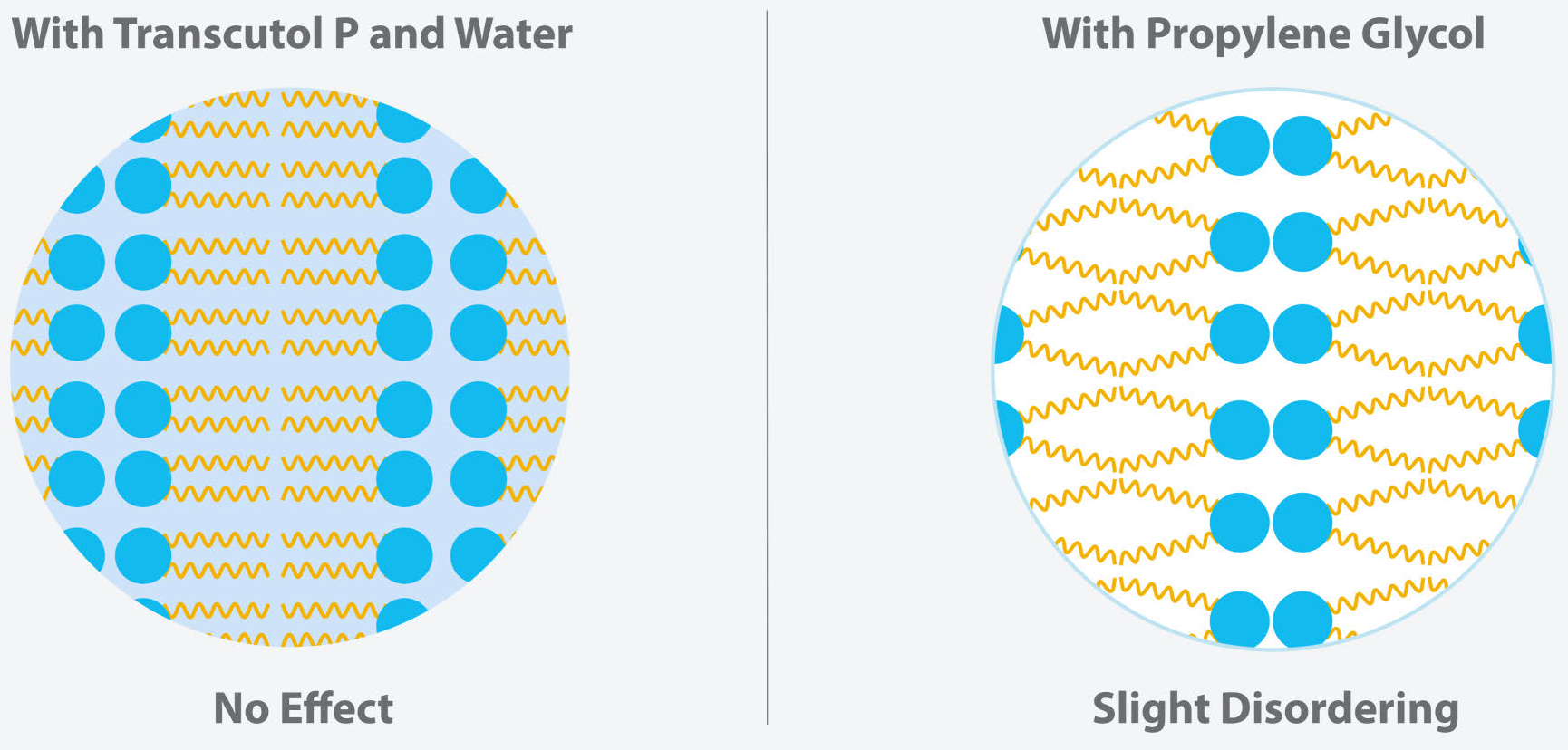
- In a Murine model, Transcutol® improved clobetsol propionate solubility and its skin retention in comparison to propylene glycol commonly used in older formulations.3
- The most common side effect of Impoyz Cream includes discoloration of the treated site. This is not the only possible side effect of Impoyz Cream.1
- Impoyz Cream can cause reversible hypothalamic-pituitary-arenal (HPA) axis suppression with the potential for glucocosticosteroid insufficiency.2
References:
1. Impoyz [package insert] Primus Pharmaceuticals, Inc. 2021.
2. Draelos Z, et al. A Randomized, Parallel Group, Open Label, Multicenter Study to Assess the Potential for Adrenal Suppression and Systemic Drug Absorption Following Multiple Dosing with Clobetasol Propionate Cream (Impoyz® ), 0.025% versus Clobetasol Propionate (Temovate®) Cream, 0.05% in Subjects with Moderate to Severe Plaque Psoriasis. Skin. 2018. 2:(6)410-420.
3. Trivedi K et al. Development of Novel Microemulsion Based Topical Formulation of Clobetasol Propionate and Salicylic Acid for The Treatment of Psoriasis. Int. Res. J. Pharm. 2018;9 (5).
4. Ghadially R, et al. Stratum Corneum Structure and Function Correlates with Phenotype in Psoriasis. Invest Dermatol. 1996; 107:558-564.
5. W. Osborne, David. Skin Penetration and Permeation Properties of Transcutol®--Neat or Diluted Mixtures. AAPS PharmSciTech. November 2018.
1. Impoyz [package insert] Primus Pharmaceuticals, Inc. 2021.
2. Draelos Z, et al. A Randomized, Parallel Group, Open Label, Multicenter Study to Assess the Potential for Adrenal Suppression and Systemic Drug Absorption Following Multiple Dosing with Clobetasol Propionate Cream (Impoyz® ), 0.025% versus Clobetasol Propionate (Temovate®) Cream, 0.05% in Subjects with Moderate to Severe Plaque Psoriasis. Skin. 2018. 2:(6)410-420.
3. Trivedi K et al. Development of Novel Microemulsion Based Topical Formulation of Clobetasol Propionate and Salicylic Acid for The Treatment of Psoriasis. Int. Res. J. Pharm. 2018;9 (5).
4. Ghadially R, et al. Stratum Corneum Structure and Function Correlates with Phenotype in Psoriasis. Invest Dermatol. 1996; 107:558-564.
5. W. Osborne, David. Skin Penetration and Permeation Properties of Transcutol®--Neat or Diluted Mixtures. AAPS PharmSciTech. November 2018.
