Want the lowest possible price on your IMPOYZ prescription?*
*Terms and conditions may apply
A TRANSFORMULATION of topical clobetasol that patients can appreciate

DIRECTIONS FOR USE: Apply a thin layer of IMPOYZ cream to the affected skin area twice daily and rub in gently and completely. Approved for use for up to 2 consecutive weeks of treatment.1
Click to get information TAILORED TO YOUR NEEDS

Putting the needs of PATIENTS FIRST
Primus makes it easier to prescribe, reducing burden for the patient and the physician in getting access to much-needed treatments.
LEARN MORE
IMPOYZ® (clobetasol propionate) Cream, 0.025%, for topical use
INDICATION AND IMPORTANT SAFETY INFORMATION
What is IMPOYZ Cream?
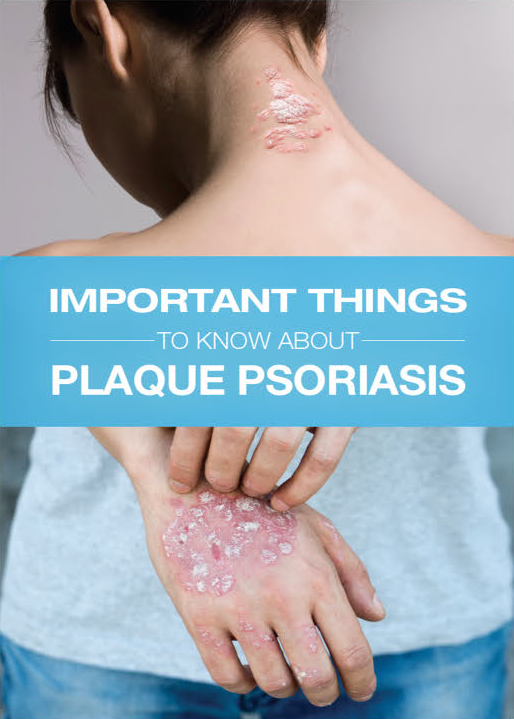
IMPOYZ Cream is a corticosteroid used to treat moderate to severe plaque psoriasis in people 18 years of age and older.
It is not known if IMPOYZ Cream is safe and effective in children under 18 years of age. IMPOYZ Cream is not recommended for use in children under 18 years of age.
IMPORTANT SAFETY INFORMATION
IMPOYZ Cream is for use on the skin only.
Do not get IMPOYZ Cream near or in your eyes, mouth, or vagina.
Before using IMPOYZ Cream, tell your doctor about all your medical conditions, including if you:
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines. Especially tell your doctor if you take other corticosteroid medicines by mouth or on your skin.
Do not use other products containing a corticosteroid medicine with IMPOYZ Cream without talking to your doctor first.
How should I use IMPOYZ Cream?
What are the possible side effects of IMPOYZ Cream?
IMPOYZ Cream may cause serious side effects, including:
The most common side effect of IMPOYZ Cream includes discoloration of the treated site. These are not all the possible side effects of IMPOYZ Cream. Call your doctor for medical advice about side effects.
You may report side effects to Primus Pharmaceuticals, Inc. at 480-483-1410 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
INDICATION AND IMPORTANT SAFETY INFORMATION
What is IMPOYZ Cream?
IMPOYZ Cream is a corticosteroid used to treat moderate to severe plaque psoriasis in people 18 years of age and older.
It is not known if IMPOYZ Cream is safe and effective in children under 18 years of age. IMPOYZ Cream is not recommended for use in children under 18 years of age.
IMPORTANT SAFETY INFORMATION
IMPOYZ Cream is for use on the skin only.
Do not get IMPOYZ Cream near or in your eyes, mouth, or vagina.
Before using IMPOYZ Cream, tell your doctor about all your medical conditions, including if you:
- have thinning of the skin (atrophy) at the treatment site.
- have a skin infection. You may need to treat the skin infection before using IMPOYZ Cream.
- have diabetes.
- have adrenal gland problems.
- plan to have surgery.
- have liver problems.
- are pregnant or plan to become pregnant. It is not known if IMPOYZ Cream will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if IMPOYZ Cream passes into breast milk.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines. Especially tell your doctor if you take other corticosteroid medicines by mouth or on your skin.
Do not use other products containing a corticosteroid medicine with IMPOYZ Cream without talking to your doctor first.
How should I use IMPOYZ Cream?
- Use IMPOYZ Cream exactly as your doctor tells you to use it.
- Your doctor should tell you how much IMPOYZ Cream you should use and where to apply it.
- Apply a thin layer of IMPOYZ Cream to the affected skin areas 2 times each day and rub in gently and completely.
- Use IMPOYZ Cream for the shortest amount of time needed to treat your plaque psoriasis. Tell your doctor if your skin condition is not getting better after 2 weeks of using IMPOYZ Cream. Do not use IMPOYZ Cream for longer than 2 weeks in a row.
- Do not use IMPOYZ Cream on your face, scalp, underarms (armpits), groin, or areas where your skin may touch and rub together.
- Do not use IMPOYZ Cream if thinning of the skin (atrophy) is present.
- Do not bandage, cover, or wrap the treated skin area unless your doctor tells you to.
- Wash your hands after you apply IMPOYZ Cream.
- See your doctor regularly to check your symptoms and side effects while using IMPOYZ Cream
What are the possible side effects of IMPOYZ Cream?
IMPOYZ Cream may cause serious side effects, including:
- Symptoms of a disorder where the adrenal gland does not make enough of certain hormones (adrenal insufficiency) during treatment or after stopping treatment with IMPOYZ Cream. Your doctor may do blood tests to check for adrenal gland problems.
- Cushing syndrome, a condition that can happen when your body is exposed to too much of the hormone cortisol. Your doctor may do tests to check for this.
- High blood sugar (hyperglycemia) or diabetes mellitus that has not been diagnosed can happen with treatment. Your doctor may do tests to check for this.
- Skin reactions at the treated skin site. Tell your doctor if you get any skin reactions or skin infections.
- Effects on growth and weight in children.
The most common side effect of IMPOYZ Cream includes discoloration of the treated site. These are not all the possible side effects of IMPOYZ Cream. Call your doctor for medical advice about side effects.
You may report side effects to Primus Pharmaceuticals, Inc. at 480-483-1410 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch